In the microscopic world of pharmaceuticals, the interface between two phases — whether solid, liquid, or gas — often decides how a drug dissolves, spreads, or stabilizes in a formulation. The invisible tension at these boundaries shapes everything from tablet coating and emulsions to detergent action and solubilization.
The study of surface and interfacial phenomena bridges chemistry and pharmacy, providing insights into how molecules behave when they meet at the boundary of two worlds.
Download UNIT 3 – Understanding Surface and Interfacial Phenomena in Pharmacy Notes
Get simplified revision notes for this unit:
⬇️
Download Unit 3 Notes PDF
The Liquid Interface: Where Two Worlds Meet
A liquid interface is the boundary between two immiscible liquids, such as oil and water, or between a liquid and air. At this boundary, molecules experience unbalanced forces — those at the surface are not surrounded equally by neighboring molecules, leading to a state of higher potential energy.
This imbalance gives rise to surface tension, the force that makes water droplets spherical and enables some insects to walk on water. In pharmaceutical science, this concept is crucial for formulating emulsions, suspensions, and foams — systems where liquids of different polarities must coexist harmoniously.
Surface and Interfacial Tensions: Balancing Molecular Forces
Surface tension refers to the force per unit length acting along the surface of a liquid, caused by molecular cohesion. Interfacial tension, on the other hand, occurs at the boundary between two immiscible liquids.
For example, the oil-water interface in an emulsion has significant interfacial tension due to the difference in polarity between the two phases. Reducing this tension — usually with the help of surface-active agents (surfactants) — allows the formation of stable emulsions, a cornerstone in pharmaceutical and cosmetic formulations.
Surface Free Energy: The Energy Behind the Interface
Every interface possesses a certain amount of surface free energy, which is the energy required to create a new surface. Molecules at the surface are at a higher energy state because they are not surrounded by similar molecules on all sides.
In practical terms, minimizing this energy helps in stabilizing formulations. The lower the surface free energy, the more stable the interface — an essential principle in drug coatings, solubilization, and colloidal stability.
Measuring Surface and Interfacial Tensions
Scientists use various methods to measure surface and interfacial tension:
Capillary Rise Method: Based on how high a liquid rises or falls in a capillary tube due to surface tension.
Drop Weight or Drop Volume Method: Determines tension by measuring the weight or volume of a drop as it detaches from a capillary.
Wilhelmy Plate and Du Noüy Ring Methods: Measure the force required to detach a plate or ring from the liquid surface.
These techniques help researchers design formulations with optimal surface properties for drug stability and bioavailability.
The Spreading Coefficient: Measuring Compatibility
The spreading coefficient (S) determines whether one liquid will spread over another to form a uniform layer. It is expressed as:
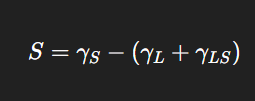
Where:
= surface tension of substrate
= surface tension of liquid
= interfacial tension between liquid and substrate
If S is positive, spreading occurs spontaneously; if negative, the liquid forms droplets. This principle governs ointments, creams, and topical drug delivery systems, where spreading ensures even distribution on the skin or mucosal surface.
Adsorption at Liquid Interfaces
Adsorption is the process by which molecules accumulate at an interface rather than in the bulk phase. In liquids, this occurs because certain molecules, especially those with hydrophilic and hydrophobic ends, migrate to the surface to lower the overall surface tension.
For instance, surfactants (like soaps and detergents) align at the liquid interface — their hydrophilic heads in water and hydrophobic tails in oil or air. This reduces tension and stabilizes emulsions and foams.
In pharmacy, adsorption at liquid interfaces influences drug solubility, emulsification, and suspension stability, making it vital in both oral and topical formulations.
Surface-Active Agents (Surfactants): The Molecular Mediators
Surface-active agents are compounds that lower surface and interfacial tensions by concentrating at the interface. They are classified based on the charge of their hydrophilic group:
Anionic surfactants (e.g., sodium lauryl sulfate) – used in detergents.
Cationic surfactants (e.g., cetyltrimethylammonium bromide) – used as antiseptics.
Nonionic surfactants (e.g., polysorbates) – used in emulsions and suspensions.
Amphoteric surfactants – act as both anionic and cationic depending on pH.
Surfactants are indispensable in pharmaceutical emulsions, foams, and solubilized drug systems, ensuring drugs remain stable and effective.
The HLB Scale: Balancing Hydrophilic and Lipophilic Nature
The Hydrophilic-Lipophilic Balance (HLB) scale quantifies the balance between the water-loving (hydrophilic) and oil-loving (lipophilic) portions of a surfactant molecule.
Surfactants with low HLB values (3–6) are more lipophilic and suitable for water-in-oil (W/O) emulsions.
Those with high HLB values (8–18) are more hydrophilic, ideal for oil-in-water (O/W) emulsions.
For example, Tween 80 (HLB ~15) is widely used in O/W emulsions, whereas Span 80 (HLB ~4.3) is preferred for W/O emulsions.
Understanding and selecting the correct HLB value is crucial in formulation design, ensuring both physical stability and therapeutic consistency.
Solubilization and Detergency
Solubilization
Solubilization refers to the process of incorporating poorly soluble substances into a micellar solution using surfactants. The hydrophobic core of the micelle traps the insoluble compound, enhancing its apparent solubility.
This principle is used in oral, topical, and parenteral drug formulations, particularly for drugs with low water solubility such as vitamins and steroids.
Detergency
Detergency is the cleansing action of surfactants — the ability to remove dirt, grease, or microorganisms from a surface. It operates through emulsification, solubilization, and dispersion mechanisms.
In pharmaceuticals, detergency finds applications in cleaning surgical instruments, laboratory glassware, and hospital environments, maintaining sterile and safe conditions.
Adsorption at Solid Interfaces
When solids come in contact with gases or liquids, molecules can adsorb onto their surface. This phenomenon is essential in tablet coatings, activated charcoal formulations, and chromatography.
There are two types of adsorption:
Physical adsorption – weak van der Waals forces.
Chemical adsorption – strong chemical bonding.
For example, activated charcoal adsorbs toxins in cases of poisoning, while chromatography techniques rely on adsorption to separate complex mixtures.
