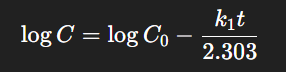Behind every medicine that reaches a pharmacy shelf lies a careful balance of science and safety — a balance ensured through drug stability. Stability testing is one of the cornerstones of pharmaceutical formulation, ensuring that a drug maintains its identity, strength, quality, and purity over time. A stable drug is not just effective but also safe for the patient throughout its intended shelf life.
This article delves into the fascinating world of reaction kinetics, degradation mechanisms, and stabilization strategies, revealing how pharmaceutical scientists keep medicines from losing their potency before they reach the patient.
Download UNIT 5 – Drug Stability: Safeguarding the Shelf Life of Medicines Notes
Get simplified revision notes for this unit:
⬇️
Download Unit 5 Notes PDF
Understanding Reaction Kinetics
Reaction kinetics deals with the rate at which chemical reactions occur and how different conditions affect these rates. In pharmaceuticals, it helps predict how fast a drug may degrade under certain environmental conditions such as heat, light, or humidity.
The rate of a reaction can be expressed as the change in concentration of a reactant or product per unit time. The rate depends on factors like temperature, concentration, solvent, and catalysts.
Zero-Order Reactions
In a zero-order reaction, the rate of degradation is independent of the concentration of the reactant. This means that the drug degrades at a constant rate, often observed in suspensions where the concentration of the dissolved drug remains constant due to the equilibrium with undissolved particles.
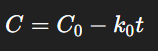
Here, k0k_0 is the zero-order rate constant, and the units are concentration/time (e.g., mol/L·s).
First-Order Reactions
In a first-order reaction, the rate of degradation is directly proportional to the concentration of the reactant. Most pharmaceutical degradation reactions follow first-order kinetics.
The rate constant k1k_1 has units of time⁻¹.
Second-Order Reactions
In second-order reactions, the rate depends on the concentrations of two reacting species, either the same or different compounds. The units of k2k_2 are L·mol⁻¹·s⁻¹.
Pseudo-Order Reactions
When one reactant is present in large excess, the reaction behaves as if it is of a lower order — this is known as a pseudo-zero or pseudo-first order reaction. Such reactions simplify kinetic analysis and are common in drug degradation processes.
Determining Reaction Order
The order of a reaction can be determined experimentally by measuring the concentration of the reactant at different times and plotting data according to zero, first, and second-order rate equations.
A linear plot of concentration vs. time indicates zero-order kinetics.
A linear plot of log concentration vs. time suggests first-order kinetics.
A linear plot of 1/concentration vs. time corresponds to second-order kinetics.
These studies help pharmacists estimate the shelf life (t₉₀) of drugs — the time it takes for 10% of the active ingredient to degrade.
Factors Influencing Drug Degradation
Drug degradation is influenced by various physical and chemical factors, which must be controlled to ensure product stability.
1. Temperature
According to the Arrhenius equation, reaction rates increase exponentially with temperature. Even a small rise in temperature can significantly accelerate degradation, which is why most stability testing involves temperature control or simulation through accelerated studies.
2. Solvent and Dielectric Constant
The choice of solvent affects reaction rates, especially in hydrolysis and oxidation. Solvents with lower dielectric constants often promote reactions between neutral molecules, while polar solvents may stabilize ionic intermediates.
3. Ionic Strength
Changes in ionic strength of the medium can alter the rate of ionic reactions by affecting electrostatic interactions between reactants.
4. Catalysis
Drugs may undergo acid-base catalysis, where hydrogen ions (H⁺) or hydroxide ions (OH⁻) speed up the reaction.
Specific catalysis involves direct participation of H⁺ or OH⁻.
General catalysis involves other acidic or basic species such as buffers.
These factors are crucial when designing formulations and choosing excipients that minimize unwanted reactions.
Stabilization of Medicinal Agents
To prevent or slow down degradation, formulators apply several stabilization strategies tailored to the specific degradation pathways.
1. Protection Against Hydrolysis
Hydrolysis — the breakdown of a compound due to reaction with water — is common in esters and amides.
To minimize it:
Replace water with non-aqueous solvents (like glycerin or ethanol).
Adjust pH to the least reactive zone.
Use desiccants and moisture-resistant packaging.
2. Protection Against Oxidation
Oxidation involves the addition of oxygen or loss of electrons, often catalyzed by light or trace metals.
It can be prevented by:
Adding antioxidants like ascorbic acid or sodium bisulfite.
Using chelating agents (e.g., EDTA) to remove metal ions.
Excluding oxygen by purging with nitrogen or using airtight containers.
3. Photolytic Degradation
Some drugs degrade under light exposure through photolysis, leading to color change or loss of potency.
To prevent this:
Use amber-colored containers.
Add light-absorbing stabilizers.
Store drugs away from direct sunlight.
Accelerated Stability Testing and Expiration Dating
Predicting how long a drug remains stable under normal conditions can take years. To shorten this process, scientists use Accelerated Stability Testing (AST).
In AST, the product is stored at elevated temperatures and humidity levels, and degradation is monitored over time. Using the Arrhenius equation, data from these tests are extrapolated to predict the shelf life under normal storage conditions.
This method allows pharmaceutical companies to set expiration dates confidently while ensuring safety and efficacy throughout the product’s marketed life.