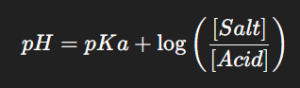In the complex and sensitive world of pharmaceuticals and biology, maintaining the right chemical balance is not just a matter of science — it’s a matter of survival. The concepts of pH, buffers, and isotonic solutions form the invisible framework that ensures stability in both drug formulations and living systems. Without these finely tuned systems, medicines could lose their potency and biological processes could falter.
Download UNIT 5 – pH, Buffers, and Isotonic Solutions — The Chemistry That Keeps Life in Balance Notes
Get simplified revision notes for this unit:
⬇️
Download Unit 5 Notes PDF
Understanding pH — The Power of Hydrogen
The term pH stands for the potential of hydrogen, and it measures the acidity or alkalinity of a solution. Introduced by Danish biochemist Sørensen in 1909, the Sørensen’s pH scale ranges from 0 to 14.
A pH of 7 represents neutrality — pure water.
Values below 7 indicate acidity, where hydrogen ion concentration [H+]is higher.
Values above 7 reflect alkalinity, dominated by hydroxyl ions [OH−].
Even a small shift in pH can drastically affect chemical reactions. For instance, in the human body, blood pH must remain within the narrow range of 7.35 to 7.45. A deviation beyond this can lead to serious physiological disorders such as acidosis or alkalosis.
Determining pH — Measuring the Invisible
Measuring pH accurately is vital in pharmaceutical manufacturing, quality control, and biological studies. Two major methods are widely used:
1. Electrometric Method
This technique uses a pH meter equipped with glass and reference electrodes. When immersed in a solution, the electrodes generate a voltage proportional to the hydrogen ion concentration. This voltage is converted into a pH reading. The electrometric method is precise, rapid, and suitable for both clear and colored solutions.
2. Colorimetric Method
This traditional method relies on acid-base indicators that change color depending on the pH. Indicators such as phenolphthalein, methyl orange, and bromothymol blue are commonly used. The color produced by the solution is compared with a standard color chart to estimate the pH. Though less accurate, it is useful for quick assessments or educational demonstrations.
Buffers — Guardians of pH Stability
In chemistry and biology alike, buffers act as protectors against drastic pH changes. A buffer is a solution that resists changes in pH when small amounts of acid or base are added.
The effectiveness of a buffer depends on two key parameters:
Buffer Equation: Described by the Henderson–Hasselbalch equation,

This equation helps in calculating the pH of a buffer solution and designing pharmaceutical formulations.
Buffer Capacity: This indicates how much acid or base a buffer can neutralize before its pH begins to change significantly. High buffer capacity is desirable in drug systems that must remain stable over time.
Buffers in Pharmaceutical and Biological Systems
In pharmaceutical science, buffers are indispensable. They ensure the stability and efficacy of drug formulations by maintaining a consistent pH.
Applications in Pharmaceuticals:
Injectable solutions must maintain a pH compatible with blood to prevent irritation or tissue damage.
Ophthalmic preparations (eye drops) use buffers to match the natural pH of tears, ensuring comfort and preventing eye irritation.
Oral formulations use buffering agents to enhance drug solubility and absorption.
Biological Importance:
In living systems, buffer systems regulate the pH of vital fluids. The bicarbonate buffer system maintains blood pH, while phosphate buffers control intracellular fluid balance. These biological buffers are nature’s way of ensuring that enzymatic reactions occur efficiently.
Isotonic Solutions — Balancing Osmotic Pressure
Alongside pH and buffers, isotonic solutions play a crucial role in pharmaceutical and physiological applications. An isotonic solution has the same osmotic pressure as body fluids such as blood or tears. This means that cells neither shrink nor swell when exposed to these solutions — maintaining equilibrium.
Buffered Isotonic Solutions
Buffered isotonic solutions combine the principles of osmotic and pH balance. They are particularly important in formulations like:
Intravenous (IV) infusions, which must match the tonicity of blood plasma.
Eye drops and nasal sprays, which prevent discomfort or irritation.
Topical and parenteral drugs, ensuring compatibility with body tissues.
A classic example is Normal Saline (0.9% NaCl) — a simple yet vital isotonic solution that matches the osmotic pressure of human plasma.
The Interplay Between pH, Buffers, and Isotonicity
In drug design and formulation, these three factors — pH, buffering capacity, and isotonicity — work together to determine how a medicine behaves in the body. For instance, if an injectable drug is too acidic or alkaline, it can cause tissue damage. Similarly, a non-isotonic solution may lead to cell rupture or dehydration.
Thus, understanding the balance among these parameters is critical to ensuring safety, comfort, and therapeutic efficiency.