As pharmacovigilance evolves, the focus is shifting from a “one-size-fits-all” approach to more personalized and population-specific safety monitoring. Pharmacogenomics, evaluation of drug safety in special populations, and harmonized regulatory frameworks now play a crucial role in understanding and preventing adverse drug reactions (ADRs). At the same time, global and national bodies such as CIOMS and CDSCO shape pharmacovigilance practices through guidelines, reporting tools, and legal requirements.
Download UNIT 5 – Pharmacogenomics, Special Populations, and Regulatory Frameworks Notes
Get simplified revision notes for this unit:
⬇️
Download Unit 5 Notes PDF
Linking Genetics to Drug Safety
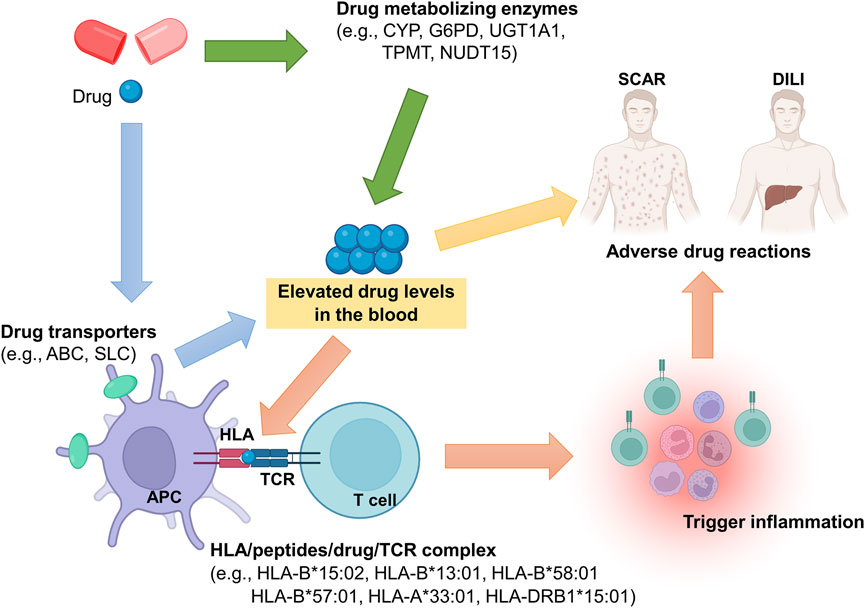
Pharmacogenomics is the study of how genetic variations influence an individual’s response to drugs. In pharmacovigilance, pharmacogenomics helps explain why some patients experience ADRs while others tolerate the same medicine without difficulty. Genetic differences can significantly affect pharmacokinetic (PK) parameters such as absorption, distribution, metabolism, and excretion.
For example, genetic polymorphisms in drug-metabolizing enzymes can alter drug clearance, leading to higher plasma concentrations and increased toxicity. Variations in cytochrome P450 enzymes, particularly CYP2D6 and CYP2C19, are well-known contributors to genetically mediated ADRs. Poor metabolizers may accumulate drugs to toxic levels, while ultra-rapid metabolizers may experience therapeutic failure or unexpected adverse effects.
Genetics-Related ADRs: Focus on Pharmacokinetics
Genetics-related ADRs are often linked to altered PK behavior. Reduced metabolic capacity can increase the area under the curve (AUC) and prolong drug half-life, raising the risk of dose-dependent toxicity. Conversely, enhanced metabolism may reduce drug exposure, leading to lack of efficacy or withdrawal-like reactions. Understanding these genetic influences allows regulators and clinicians to recommend dose adjustments, genetic testing, or alternative therapies, thereby reducing preventable ADRs.
Drug Safety Evaluation in Special Populations
Paediatrics: Safety in Developing Systems
Drug safety evaluation in paediatric populations presents unique challenges because children are not simply “small adults.” Immature organ systems, particularly the liver and kidneys, affect drug metabolism and elimination. As a result, pharmacovigilance in paediatrics emphasizes age-appropriate dosing, formulation suitability, and careful monitoring of growth and development-related adverse effects.
Post-marketing surveillance is especially important in paediatrics, as many medicines are used off-label due to limited clinical trial data in children.
Pregnancy and Lactation: Protecting Two Lives
Safety evaluation during pregnancy and lactation is critical because drug exposure can affect both the mother and the fetus or infant. Physiological changes during pregnancy alter pharmacokinetics, potentially increasing or decreasing drug exposure. Pharmacovigilance systems closely monitor outcomes such as congenital anomalies, fetal growth restriction, and neonatal toxicity.
Pregnancy registries and post-marketing studies are essential tools for collecting safety data in this population, as ethical considerations often limit controlled clinical trials.
Geriatrics: Managing Polypharmacy and Vulnerability
The geriatric population is particularly vulnerable to ADRs due to age-related physiological changes, comorbidities, and polypharmacy. Reduced renal and hepatic function can impair drug elimination, increasing the risk of accumulation and toxicity.
Pharmacovigilance in geriatrics focuses on identifying drug–drug interactions, dose adjustments, and preventable ADRs associated with inappropriate prescribing. Real-world safety data plays a vital role in optimizing therapy for elderly patients.
CIOMS and Global Pharmacovigilance Harmonization
Role of CIOMS in Drug Safety
The Council for International Organizations of Medical Sciences (CIOMS) works in collaboration with the World Health Organization (WHO) to harmonize global pharmacovigilance practices. CIOMS develops internationally accepted guidance on drug safety monitoring, reporting, and risk management.
CIOMS Working Groups and Their Contributions
CIOMS Working Groups address specific pharmacovigilance challenges, such as benefit–risk assessment, signal detection, and safety communication. Their recommendations often influence regulatory guidelines worldwide, promoting consistency in how safety data is collected and interpreted.
CIOMS Form: Standardized Safety Reporting
The CIOMS Form is a standardized template for reporting individual case safety reports (ICSRs). It ensures uniform collection of essential information, including patient details, suspected drug, adverse event description, and outcome. The CIOMS Form has become a global benchmark for spontaneous ADR reporting.
CDSCO (India) and Pharmacovigilance Regulation
Indian Regulatory Framework for Drug Safety
In India, pharmacovigilance is regulated by the Central Drugs Standard Control Organization (CDSCO) under the Ministry of Health and Family Welfare. CDSCO oversees drug approval, clinical trials, and post-marketing safety monitoring, ensuring compliance with national regulations.
Drugs and Cosmetics Act and Schedule Y
The Drugs and Cosmetics (D&C) Act provides the legal foundation for drug regulation in India. Schedule Y specifically outlines requirements for clinical trials, safety reporting, and pharmacovigilance obligations. It mandates reporting of serious adverse events and emphasizes ethical conduct and patient safety throughout the drug lifecycle.
Differences Between Indian and Global Pharmacovigilance Requirements
Harmonization with Regional Variations
While Indian pharmacovigilance requirements are increasingly aligned with global standards, differences remain in reporting timelines, documentation formats, and regulatory procedures. Global frameworks, guided by international bodies, often emphasize electronic reporting and extensive risk management planning, whereas India continues to strengthen infrastructure and capacity through national programmes.
Understanding these differences is essential for pharmaceutical companies operating in both Indian and international markets to ensure regulatory compliance.